[Download the PDF of Chemical Nomenclature Rules here.]
What Are Polyatomic Ions?
Unlike monoatomic ions, which are composed of a single atom, polyatomic ions are composed of many atoms. (The prefix poly- means “many,” as in polygon or polytheism.)
There are some rules and patterns which can help us to deduce the structures of these ions, but before we can learn those, there are a handful that don’t follow the patterns that you just need to memorize. Depending on your course or instructor, you may need to memorize some of the following. When it comes to memorizing the, flashcards are your friends!
| Cation | Formula | Anion | Formula | Anion | Formula |
| NH4+ | ammonium ion | C2H3O2– | acetate ion | C2O42- | oxalate ion |
| H3O+ | hydronium ion | CrO42- | chromate ion | MnO4– | permanganate ion |
| CN– | cyanide ion | O22- | peroxide ion | ||
| Cr2O72- | dichromate ion | S2O32- | thiosulfate ion | ||
| OH– | hydroxide ion |
The good news, is there are dozens of other polyatomic ions that are relatively easy to figure just by knowing or observing a few patterns.
The -ate Ions
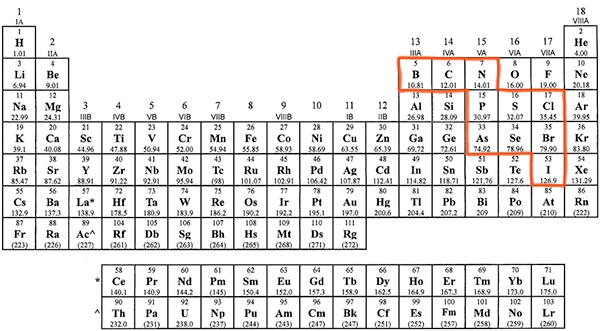
Many of the nonmetals combine with oxygen to make anions (negatively charged ions) that we are sometimes referred to as oxyanions. If you refer to the periodic table below, you will that a several nonmetals have been highlighted that commonly form such oxyanions.
These nonmetals for polyatomic ions that end in the suffix -ate. However, the number of oxygens and the charge on the ions varies as we can see in the following table.
| BO33- borate ion |
CO32- carbonate ion |
NO3– nitrate ion |
||
| PO43- phosphate ion |
SO42- sulfate ion |
ClO3– chlorate ion |
||
| AsO43- arsenate ion |
SeO42- selenate ion |
BrO3– bromate ion |
||
| IO3– iodate ion |
Patterns for Polyatomic Oxyanions
- Notice how the four polyatomic-ate ions in the center square (phosphate, arsenate, sulfate, and selenate) all have four oxygen atoms, while the polyatomic -ate ions on the outside all have three oxygen atoms.
- As you start from right-hand side, the first column of polyatomic -ate ions (chlorate, bromate, iodate) all have a 1- charge. The second column (sulfate, selenate) all have a 2- charge, while the third column (phosphate, arsenate) all have a 3- charge. On the top row, the farthest right polyatomic -ate ion is nitrate which is 1-, followed by carbonate which is 2-, followed by borate which is 3-.
- Within a column, the pattern also repeats. For example, if you know that chlorate is ClO3–, then you can predict that all of the other oxyanions made from nonmetals in group 17/VIIA follow the same pattern. Cl combines with three oxygens and has a 1- charge; Br combines with three oxygens and has a 1- charge; I combines with three oxygens and has a 1- charge. In group 16/VIA, both S and Se have the same pattern. In group 15/VA, P and As have the same pattern, but notice N is different, combining with only three oxygens and having only a 1- charge.
Changing the Number of Oxygens
Once you know the polyatomic ions that end in the suffix -ate there are only a few more patterns to know and you’ll be on your way to naming dozens and dozens of polyatomic ions!
| Increasing # Of Oxygen Atoms |
Prefix | Root | Suffix | |
| +1 O atom | per- | root | -ate | |
| – | root | -ate | ||
| -1 O atom | root | -ite | ||
| -2 O atom | hypo- | root | -ite |
For example, let’s use chlorate as an example. We know that chlorate is ClO3–. This is the anion that ends in -ate. Let’s look at the chart above and see if we can figure out what perchlorate would be. Since it has the prefix per- and the suffix -ate, it must have one O atom more than chlorate, meaning the formula is ClO4–. Notice only the number of oxygen atoms changed, the charge did not.
What would chlorite be? The suffix -ite means it has one O atom less than chlorate, so it must be ClO2–.
How about hypochlorite? The prefix hypo- and the suffix -ite mean it has two less O atoms than chlorate, so it must be ClO–.
We can similarly predict sulfite and hyposulfite once we know the formula and charge of sulfate. (Ignore persulfate as it’s an exception to the general rule.)
| Name | Formula | Name | Formula |
| perchlorate ion | ClO4– | ||
| chlorate ion | ClO3– | sulfate ion | SO42- |
| chlorite ion | ClO2– | sulfite ion | SO32- |
| hypochlorite ion | ClO– | hyposulfite ion | SO22- |
Notice that the prefixes and suffixes don’t denote the total number of O atoms, just that it has more or less than the -ate polyatomic ion.
Example Problems
Predict the formula and charge for:
1. sulfite ion – We determine by sulfite is by first figuring out what sulfate. Sulfate comes from sulfur which is in the center box of the nonmetals so it have four oxygens. It’s two columns away from the right-hand side so its charge is 2-. Sulfate is SO42-. The suffix -ite tells me it lost one oxygen atom, so sulfite must be SO32-.
2. hypophosphite ion – Let’s figure out what phosphate is. The root comes from phosphrous which is in the center box of nonmetals so it must have four oxygen atoms. Phosphrous is three columns away from the right-hand side so its charge is 3-. Therefore, phosphate is PO43-. The prefix hypo- and the suffix -ite tell me to subtract two oxygen atoms, so hypophosphite must be PO23-.
3. perbromate ion – Bromate comes from bromine which is outside the center box of nonmetals, so it combines with three oxygens. It’s in the first column from the right so its charge is 1-. Bromate must be BrO3–. The prefix per- tells me to add an oxygen, so perbromate must be BrO4–.
4. nitrite ion – Nitrate is NO3– and the -ite suffix means I must remove an oxygen atom, so nitrite must be NO2–.
How would you name the following:
5. IO2– – Let’s determine what the -ate version of iodine’s polyatomic is. Iodine is outside the center box, so it must have three oxygen atoms. Iodine is in the first column from the right-hand side so the charge on the polyatomic must be 1- (or just -). Therefore, iodate must be IO3–. However, this polyatomic has one less oxygen atom which means the suffix changes to -ite, so we would name it iodite ion.
6. AsO23- – Arsenate would be AsO43- and this has two fewer oxygen atoms. According to our rules, two less oxygens means we add the prefix hypo- and change the suffix to -ite, so this would be the hypoarsenite ion.
7. SeO32– – Selenate is SeO42-. The given ion has one less oxygen atom, so we must change the suffix from -ate to -ite. Therefore, we would name this the selenite ion.
“Hydrogen” Polyatomic Ions
Another way to form polyatomic ions is by combining them with one or more hydrogen ions, H+. For example, we could combine H+ with carbonate, CO32- to form hydrogen carbonate, HCO3–. Notice the overall charge is 1- because the 1+ on H+ combine with the 2- on CO32-.
What would hydrogen sulfate be? “Hydrogen” tells me to add H+ to sulfate, SO42-. So hydrogen sulfate would be HSO4–.
When we add hydrogen ions to a polyatomic with a 3- charge, such as phosphate (PO43-), we could add either one or two H+ ions and it would still remain a polyatomic ion. (Adding three H+ would completely cancel out the 3- charge making it a neutral compound and no longer a polyatomic ion.) We have two options then, HPO42- and H2PO4–. The former has only one hydrogen ion so call it either hydrogen phosphate ion, or sometimes monohydrogen phosphate ion. The latter has two hydrogen ions so we refer to it as dihydrogen phosphate ion.
Example Problems
Predict the formula and charge for:
8. hydrogen hyposulfite – Sulfate is SO42- so hyposulfite tells me to reduce the number of oxyen atoms by two, leaving SO22-. Adding a hydrogen ion, H+, it becomes HSO2–.
9. hydrogen selenide – The suffix -ide on selenide tells me the ion probably is a monoatomic anion formed from a nonmetal, in this case from selenium. Selenium is two columns away from the noble gases so it needs two electrons, meaning selenide is Se2-. If I add H+ to this, I get HS–.
10. dihydrogen borate – Borate is BO33-. Dihydrogen tells me to add two H+ ions, which produces H2BO3–.
How would you name:
11. HSO4– – If I remove an H+ from the ion, I am left with SO42-. (The charge becomes 2- because I removed a positive hydrogen ion.) SO42- is named sulfate so this ion combined with hydrogen would be hydrogen sulfate ion.
12. H2AsO3– – Removing two H+ ions leaves me with AsO33-. I know that arsenate is AsO43- and this has one less oxygen atom, so it must be aresnite. I’ve added two hydrogens to the polyatomic ion, so the result is dihydrogen arsenite ion.